In science you are constantly working with numbers, whether its the amount of a substance needed or calculating your results. Because of this we use something called Significant digits. You might be asking, What is significant digits? Well significant digits is any digit of a number that is known with certainty. Significant digits are used for accuracy and precision, when dealing with numbers. Very straight forward because we are looking for the most accurate and precise of answers.
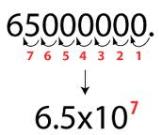
So, whats so big about significant digits? Just think about it, scientists needed to be as close as possible when they were studying about the elements. For example, when they were trying to figure out the correct atomic weight. We wouldn’t want some of the biggest foundations or discoveries to be merely a guess, we want to get as close as possible to the right amount of anything. Using significant figures is also easier when doing calculations, such as 1 mole= 6.02×10^23 is much simpler to write, instead of 1 mole=602000000000000000000000. Thank goodness for significant figures!
- Comment
- Reblog
-
Subscribe
Subscribed
Already have a WordPress.com account? Log in now.
I liked your explanation of significant numbers it was very well worded. Just don’t forget to link your sources.
Thank you very much! And I will be sure to remember sources next time.