Today I get to be the teacher, and I am going to teach you all about percent composition. Percent composition is a process where a sample of a material is analyzed by its composition. You can find the percentage of the mass of a compound that comes from each of the elements in a compound by using a simple equation. The equation is: percent of element= (number of atoms)(atomic weight) divided by FW of the compound x100.
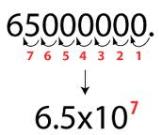
 The picture is a better visualization of what I wrote above.
The picture is a better visualization of what I wrote above.
An example of when you would use percent composition, is lets say, your trying to find the percent/amount of carbon in ethane. The formula for ethane is C2H6. You find the atomic mass of carbon, which is 12 and the atomic mass of hydrogen, which is 1. Then you know you have two carbons and six hydrogen’s by the subscripts. You add the two masses together, 2(12)+6(1) and it totals to 30. So your total mass of ethane is 30g. Now since you want to know just the percent of carbon, this is where percent composition comes in. Essentially percent composition is part over whole x100. The part we want is carbon and that is 24.0g and we know our whole is 30g. 24/30=0.8 and you multiply that times 100 and get 80. We now know that ethane is 80% carbon. Pretty easy right?